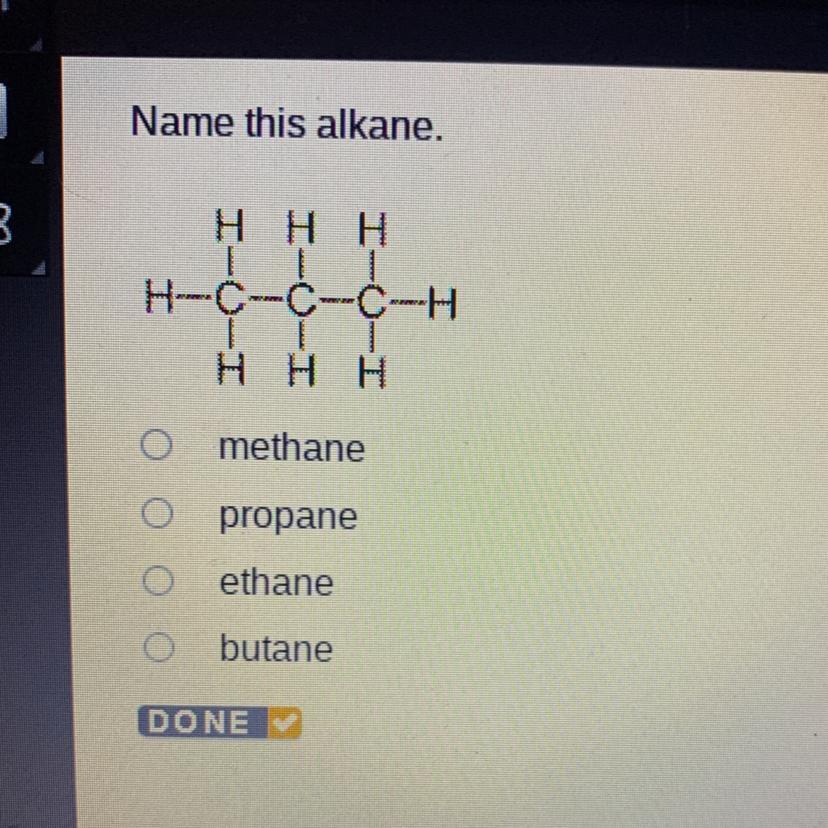
Answers
Answer:
propane
Explanation:
Related Questions
two differences between weather and climate
subject-science
Answers
Answer:
weather- rain, hail, snow, sunny, dry, etc.
climate- warm, cold, humid, dry, etc.
Explanation:
The difference if weather and climate is that climate is the way the temperature is, whether it's warm, cold, humid, dry. While weather is the state of the atmosphere at a place and time as regards heat, dryness, sunshine, wind, rain, etc.
Can someone plss help me answer those 4 questions by tonight.Thank you !
Answers
Answer:
no problem hey I will help you
→ Search the internet to understand the reaction between (Zno) & (HNO3).
a) Describe what happens to the first spoonful of powder when he mixes it with the acid.
b) Describe what would happen if the student kept adding zinc oxide to the nitric acid
after the reaction between the acid and metal oxide was complete.
Answers
Answer:
See Explanation
Explanation:
The equation of the reaction is;
ZnO(s) + 2HNO3(aq) --------> Zn(NO3)2(aq) + H2O(l)
a) When you first add ZnO to aqueous HNO3, the powder dissolves as it mixes with the acid.
b) If the student keeps on adding more ZnO powder to the HNO3 after the reaction between the acid and metal oxide was complete, the powder will no longer dissolve in the acid. At this point, the reaction has attained equilibrium.
What is the percent by mass of aspartame in iced tea that has 0.75 g of aspartame in 250 g of water?
Answers
Answer:0.30%
Explanation:
A student needs to dilute a 0.25 M Pb(NO3)2 solution to make 73.0 mL of 0.13 M Pb(NO3)2 . Set up the calculation by placing the values with the correct units into the equation. Then, calculate the volume, in milliliters, of the 0.25 M Pb(NO3)2 solution that is needed.
Answers
Answer: Volume of 0.25 M solution is 38 ml
Explanation: Amount of substance n remains same.
n = c1V1 = c2V2 Solve V2 = V1c1 / c2 = 73,0 ml · 0.13 M / 0.25 M = 37.96 ml
4. Which of the following process is NOT part of wool extraction?
(a) Shearing (b) Scouring (c) Sorting
(d) Reeling
Answers
Answer:
Reeling is the only process not listed.
The correct answer is option D: Reeling.
Wool is obtained from sheep. The wool so obtained is processed according to the following flow chart;
Shearing → Scouring → Sorting → Dyeing → Straightening, Rolling and Combing
Shearing is the removal of the fleece and thin skin of the sheep. Scouring is the process of washing the hair to remove grease, dust, and dirt. Sorting is the process of differentiating the fibers.
Hence, reeling is not a process in wool extraction.
https://brainly.com/question/9968125
What is science..
No Spam
Answers
Answer: science is a systematic enterprise that builds and organizes knowledge in the form of testable explanations and predictions about the universe
Explanation:
Answer:
Science is a very Idiot subject and doing dimag ka dahi xD
Consumers must eat other organisms
for energy. Which organisms
are consumers in this food chain?
Answers
Answer:
frog-snake+-Eagle
Explanation:
___&&&-(_(-7&&((-&(_((-_(_-8&:"&&7+_&(&_
Ionic, metallic, or covalent??? Need now
Answers
Answer:
Covalent
Explanation:
The primary forces of attraction between water molecules in H2O(l) are
1.
ionic bonds
2.
hydrogen bonds
3.
molecule-ion attractions
4.
van der Waals forces
Submit Answer
Answers
Answer:
2. Hydrogen Bonds
Explanation:
Since water is a polar covalent molecule, there is a slight negative and positive end. Due to this, the oxygen end of one water molecule gravitates towards the hydrogen molecules of another water molecule. This accounts for a bunch of weird properties of water, like why ice floats. It's also what makes water the "universal solvent," and gives all life on earth the ability to even exist.
The primary forces of attraction between water molecules in H₂O (I) are hydrogen bonds.
What kind of chemical bonding is present in water molecule?Hydrogen bonding is present in water molecule due to which it exhibits an excellent property of adhesion to itself and to other substances.The hydrogen bonding is a result of electrostatic forces of attraction which are generated by the difference in charge between slightly positive hydrogen ions and slightly negative other ions.
In case of water,hydrogen bonds are formed between neighboring hydrogen and oxygen atoms of the nearby water molecules.The attraction between water molecules itself results in a formation of a bond called as a hydrogen bond.
It is a type of covalent bond which is formed between hydrogen and oxygen atoms as one oxygen atom shares its two electrons with two hydrogen atoms .The positive charge of one hydrogen atom associates with negative charge of oxygen atom.These are weak interactions which are formed between a hydrogen atom each with a partial positive charge and an oxygen atom which is more electronegative than hydrogen.
To learn more about bonding in water click here:
https://brainly.com/question/5302822
#SPJ2
A student is asked to determine the identity of an unknown metal. The student decides to use a calorimetry experiment to find the specific heat. The student will use this as a means of identifying the metal Which of the following measurements cannot made using standard laboratory equipment?
A mass of water
B temperature of metal
с heat lost by metal
D mass of metal
Answers
Answer:
Temperture of the medal
Explanation:
Bc ik
What is a solution?
A. The substance that is dissolved in another substance
B. The mixture of one substance dissolved in another
C. The substance that dissolves another substance
D. Two liquids that do not mix with each other
SUR
Answers
Answer:
B
Explanation:
this is because solution is the mixture of a solvent and a solute to give you a solution
Answer:
B. The mixture of one substance dissolved in another
Explanation:
Using Reaction A, how many grams of CO2 can be created from 5.67 moles of water?
127.6 g CO2
199.6 g CO2
81.65 g CO2
311.85 g CO2
Answers
Answer:
81.65 g CO2
Explanation:
to the nearest gram, what is the mass of of one spoonfull of sugar? g
Answers
Answer:
the mass of one spoonful of sugar to nearest gram is 10g
Which of the following contains the least amount (number) of molecules?
Group of answer choices
5.0 g O2
5.0 g H2O
5.0 g N2
5.0 g CO2
Answers
Has to be correct
Suppose Gabor, a scuba diver, is at a depth of 15m15m. Assume that: The air pressure in his air tract is the same as the net water pressure at this depth. This prevents water from coming in through his nose. The temperature of the air is constant (body temperature). The air acts as an ideal gas. Salt water has an average density of around 1.03 g/cm3g/cm3, which translates to an increase in pressure of 1.00 atmatm for every 10.0 mm of depth below the surface. Therefore, for example, at 10.0 mm, the net pressure is 2.00 atmatm. What is the ratio of the molar concentration of gases in Gabor's lungs at the depth of 15 meters to that at the surface
Answers
Answer:
The ration of molar concentration is "2.5".
Explanation:
The given values are:
Average density of salt water,
= [tex]1.03 \ g/cm^3[/tex]
Net pressure,
= [tex]2.00 \ atm[/tex]
Increase in pressure,
= [tex]1.00 \ atm[/tex]
Now,
The under water pressure will be:
= [tex]\frac{15 \ m}{10}\times 1 \ atm +1 \ atm[/tex]
= [tex]1.5\times 1+1[/tex]
= [tex]1.5+1[/tex]
= [tex]2.5 \ atm[/tex]
hence,
The ratio will be:
= [tex]\frac{(\frac{n}{V})_{15m} }{(\frac{n}{V})_{surface} }[/tex]
or,
= [tex]\frac{P}{P_s}[/tex]
= [tex]\frac{2.5}{1}[/tex]
= [tex]2.5[/tex]
When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic cell in which solid lead(II) sulfate PbSO4 is reduced to lead at the cathode and oxidized to solid lead(II) oxide PbO at the anode. Suppose a current of 17.0A is fed into a car battery for 15.0 seconds. Calculate the mass of lead deposited on the cathode of the battery. Be sure your answer has a unit symbol and the correct number of significant digits.
Answers
Answer:
0.273504 grams
Explanation:
Charge Deposited = Current ×Time
q=It
Given: I= 17.0A, t= 15.0 seconds
Q= 17×15= 255 C
Since, 1 mole contains 96500C of charge deposition
Therefore, number of moles = 255/96500 = 0.00264 moles
Moreover, since Pb changes to Pb2+,
Hence number of moles of Pb deposited = 0.00264/2
= 0.00132
Also, Molar mass of Lead = 207.2 gm/mol
Therefore, the mass of lead deposited = 0.00132×207.2 = 0.273504 grams
.
A solution of dispersant is made by taking 15.0 mL of a 50.0 mg/mL solution of Randyne and mixing it with 50.0 mL of water. Calculate the final concentration of the Randyne in this solution, in units of grams per milliliter.
Answers
Answer:
The final concentration of the Randyne in grams per milliliter = 0.011 g/mL
Explanation:
As we know
C1V1 = C2V2
C1 and C2 = concentration of solution 1 and 2 respectively
V1 and V2 = Volume of solution 1 and 2 respectively
Substituting the given values, we get -
[tex]50 * 15 = X * (15+50)\\X = 11.54[/tex] mg/mL
The final concentration of the Randyne in grams per milliliter = 0.011 g/mL
The final concentration of the Randyne in this solution is 0.01 g /mL.
How to calculate dilutions?It is very important to know the dilution methods in a chemistry lab. The dilution from the stock solution can be prepared by using the formula,
[tex]C_1V_1 = C_2V_2[/tex]
Where,
[tex]C_1[/tex]- concentration of the stock solution
[tex]V_1[/tex] - the volume of the stock solution
[tex]C_2[/tex] - concentration of the diluted solution
[tex]V_2[/tex] - the volume of diluted solution
Put the values in the formula,
[tex]50 \times 15 = C_2 \times 75 \\\\C_2 = \dfrac {750}{75 }\\\\C_2 = 10{\rm \ mg/mL \ \ \ or} \\\\ C_2 = 0.01 \rm \ g/mL[/tex]
Therefore, the final concentration of the Randyne in this solution is 0.01 g /mL.
Learn more about dilution methods:
https://brainly.com/question/25307719
Cómo escribir el símbolo Theta
Answers
Which of the following compounds would have the lowest
solubility?
ОНczА
O Nm3R
O Bv3(AX)2
O Hn(EX2)2
O MRD2
Answers
The blank
and
blank
of atoms are the same on both sides of
a chemical equation.
Please just answer the question directly instead of giving me some weird cryptic answer that I can’t use. I’ve seen this exact question answered before and nobody would give a straight answer.
Answers
What is the name of Na
Answers
mdmsjdkskdkskdjxjxjjz
Which best describes the conservation of energy as a pendulum swings in the path shown ?
A.The potential energy at point A is greater than the potential energy at point C.
B.The potential energy at point A is equal to the kinetic energy at point C.
C.The potential energy at point A is greater than the kinetic energy at point B.
D.The potential energy at point A is equal to the kinetic energy at point B.
Answers
how to write an article?
Answers
Answer:
essay typer.com click the first link
Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a primary standard. It has the unwieldy formula of KHC8H4O4. This is often written in shorthand notation as KHP. If 31.0 mL of a barium hydroxide solution are needed to neutralize 1.37 grams of KHP, what is the molarity of the barium hydroxide solution
Answers
Answer:
0.1082M of Barium Hydroxide
Explanation:
KHP reacts with Ba(OH)2 as follows:
2KHP + Ba(OH)2 → 2H2O + Ba²⁺ + 2K⁺ + 2P²⁻
Where 2 moles of KHP reacts per mole of barium hydroxide
To solve this question we must find the moles of KHP in 1.37g. With these moles and the reaction we can find the moles of Ba(OH)2 and its molarity using the volume of the solution (31.0mL = 0.0310L) as follows:
Moles KHP -Molar mass: 204.22g/mol-
1.37g * (1mol / 204.22g) = 0.006708 moles KHP
Moles Ba(OH)2:
0.006708 moles KHP * (1mol Ba(OH)2 / 2mol KHP) =
0.003354 moles Ba(OH)2
Molarity:
0.003354 moles Ba(OH)2 / 0.0310L =
0.1082M of Barium Hydroxide1st law of motion law of inertia in toy story 2
Answers
What is The metric unit for volume ?
Answers
Answer:
milliliters
Explanation:
Is going to be milliliters because in the metric system of measurement,the most common unit of volume are milliliters and liters
Do frogs start their life cycle on land or water?
Answers
The answer is water because they lay eggs in water
What is the percent by mass of magnesium in a 1000 g sample of ocean water (solution) that has 1.36 g of magnesium ions?
Answers
Answer:
So the percentage mass of Magnesium in ocean water is 0.13%.
Explanation:
brainliest pls
Draw the most stable form of the major product in the reaction. 2 equivalents of an ester react with N a O C 2 H 5, followed by H 3 O plus and C 2 H 5 O H. The ester is a carbonyl bonded to a 3 carbon chain and O C H 2 C H 3.
Answers
Answer:
See answer below
Explanation:
This is a Claisen condensation. In the picture below you have the mechanism and final product.
Hope this helps